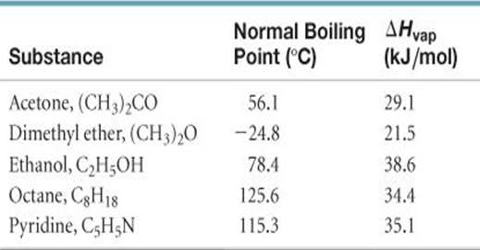
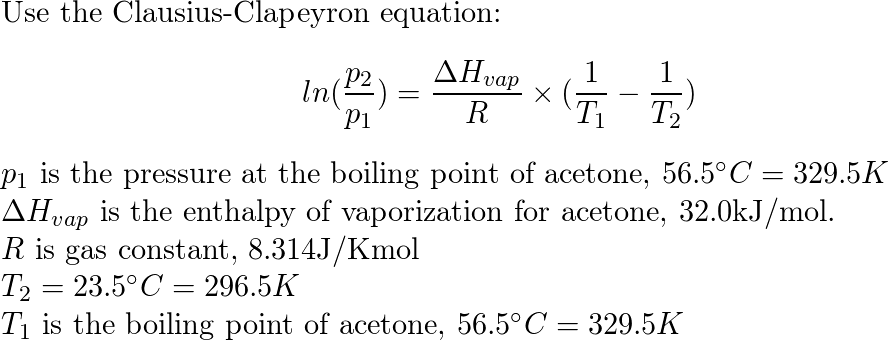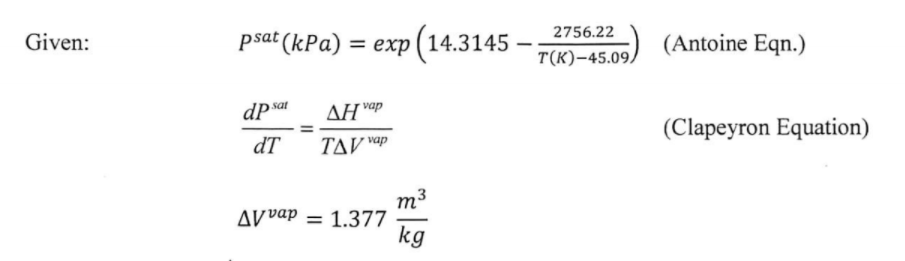Ch11-ClausiusClapeyron 2 .docx - Clausius-Clapeyron equation and heat of vaporization adapted from Dr. Sushilla Knottenbelt NAMES: 1a. In order | Course Hero

OneClass: 2) The normal boiling point of acetone is 56.5°C At an elevation of 5300 ft, the atmospher...

The molar enthalpy of vaporisation of acetone is less than that of water. Why ? | CLASS 11 | THE... - YouTube

Heat of vaporization of acetone. Simulation data: • this work, AUA4... | Download Scientific Diagram

If the normal boiling point of acetone is 56^∘ C and it has a Δ H^∘vap of 32.1 kJ/mol, estimate the boiling point at 5 bar?
How to calculate the vapor pressure of acetone at 25.0°C if the enthalpy of vaporization for acetone is 32.0 kJ/mol and the normal boiling point of acetone is 56.5°C - Quora

OneClass: A standard entropy of vaporization of acetone is approximately 85jk/mol at its boiling poin...

SOLVED: How much heat is required to vaporize 38.5 g of acetone (C3H6O; molar weight 58.1 g) at 25 °C? The heat of vaporization for acetone at this temperature is 31.0 kJ/mol.

Heat of vaporization of acetone. Simulation data: • this work, AUA4... | Download Scientific Diagram
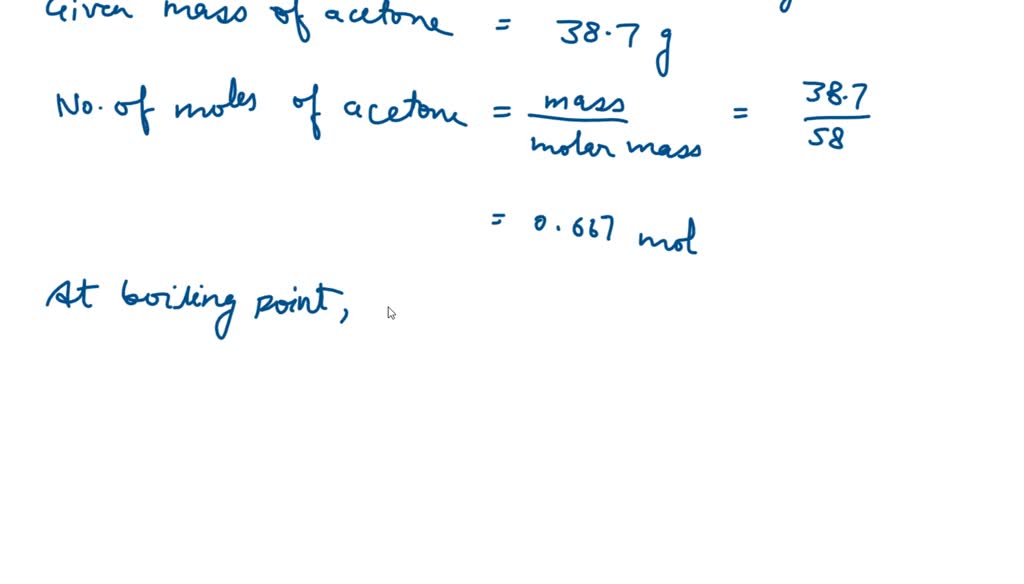
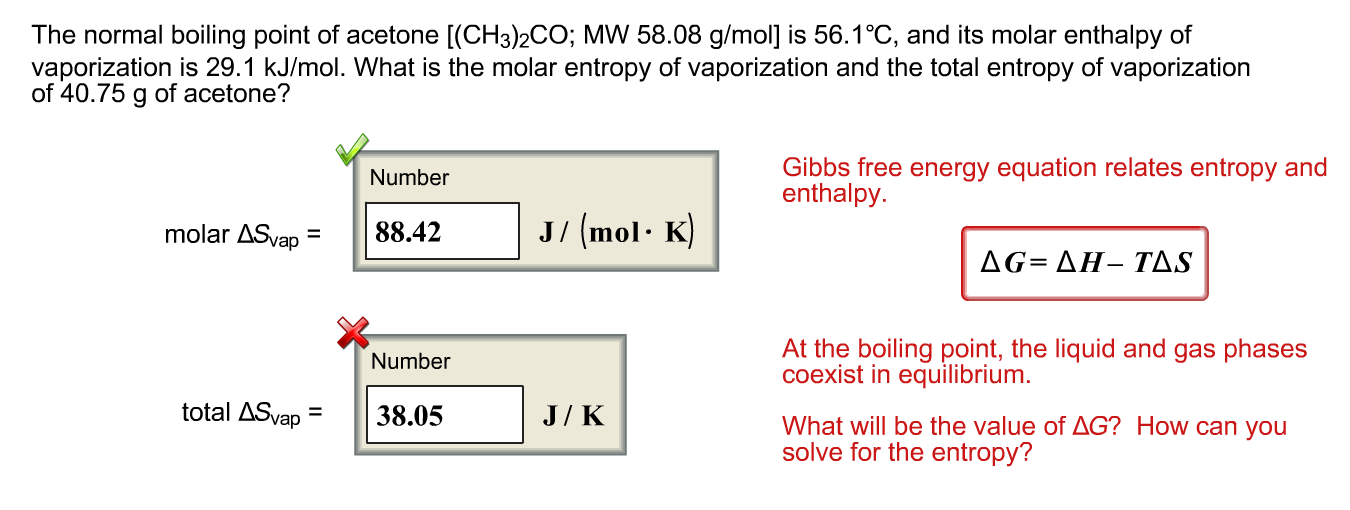
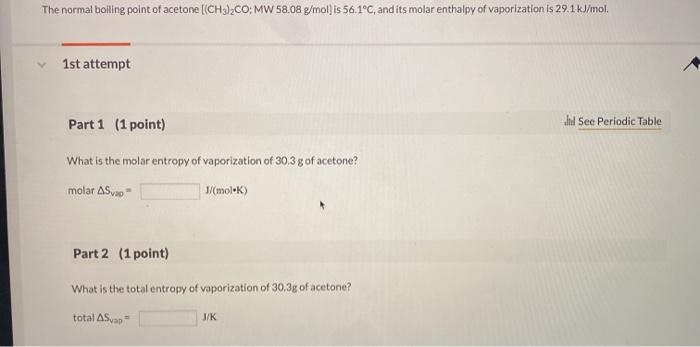
SOLVED: The heat of vaporization of acetone at its boiling point is 29.1 kJ/mol. How much energy (in kJ) do I need to add to vaporize 3.00 moles of acetone?

SOLVED:The enthalpy of vaporization for acetone is 32.0 kJ / mol . The normal boiling point for acetone is 56.5^∘ C . What is the vapor pressure of acetone at 23.5^∘ C ?